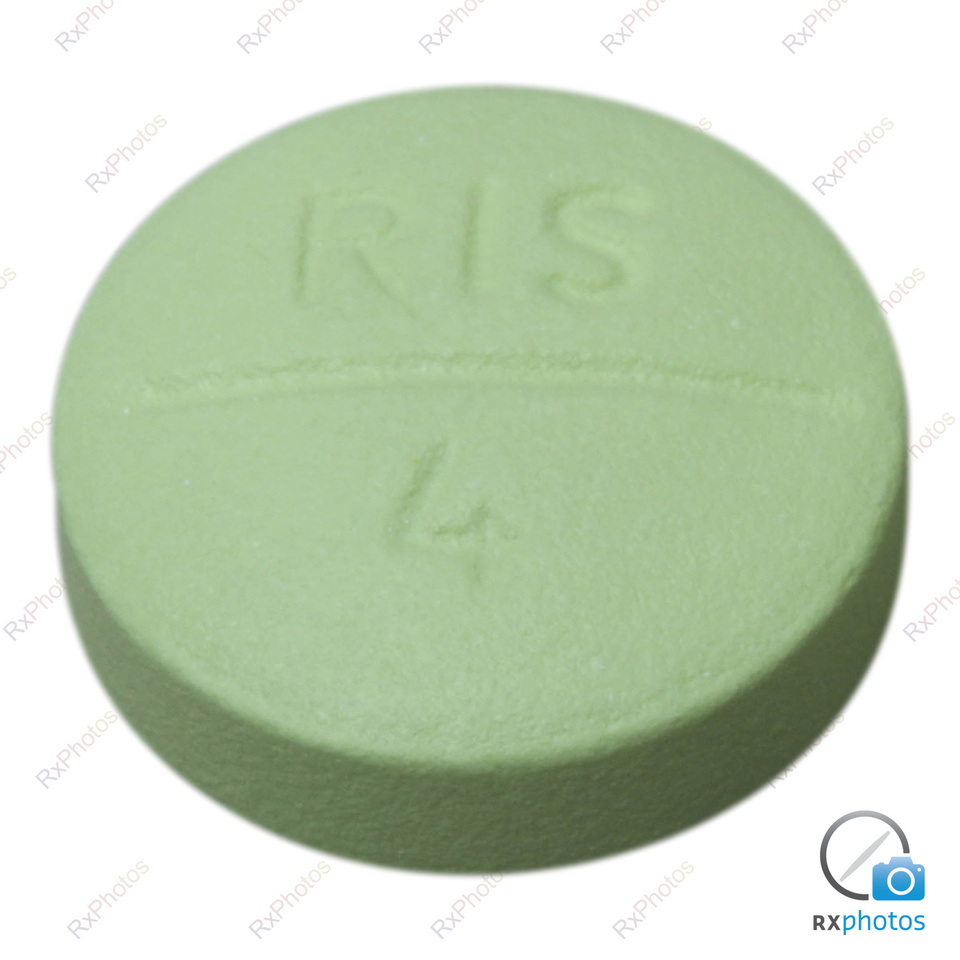
Risperidone "Teva GmbH" Teva GmbH pulver og solvens til depotinjektionsvæske, suspension 25 mg, 37,5 mg og 50 mg

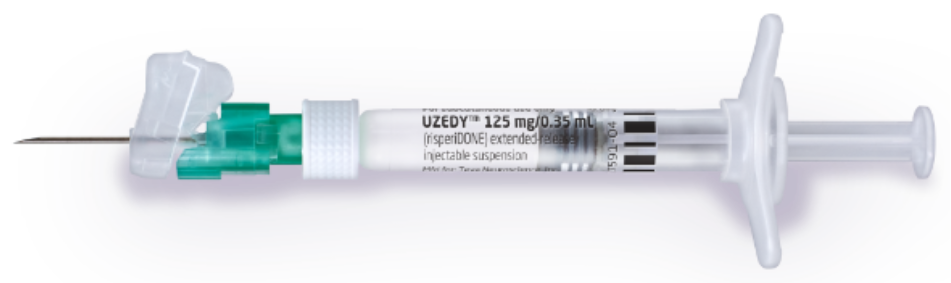
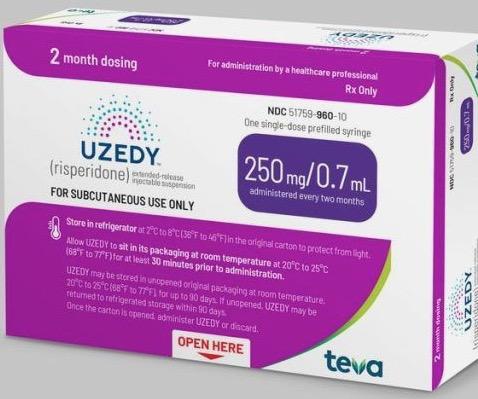
FDA Approves Uzedy (risperidone) Extended-Release Injectable Suspension for the Treatment of Schizophrenia in Adults-CliniExpert

FDA declines to approve Teva-MedinCell's risperidone injection for treating schizophrenia | Seeking Alpha

Teva and MedinCell Announce FDA Acceptance of New Drug Application for TV-46000/mdc-IRM as a Treatment for Patients with Schizophrenia | Business Wire

Teva and MedinCell Announce FDA Approval of UZEDY™ (risperidone) Extended-Release Injectable Suspension, a Long-Acting Subcutaneous Atypical Antipsychotic Injection, for the Treatment of Schizophrenia in Adults

Teva and MedinCell Announce FDA Approval of UZEDY™ (risperidone) Extended-Release Injectable Suspension, a Long-Acting Subcutaneous Atypical Antipsychotic Injection, for the Treatment of Schizophrenia in Adults
La petite pharmacie de Nanterre - Médicament Risperidone Teva Sante 1 Mg/ml, Solution Buvable - NANTERRE